This week’s blog post (aside from referencing THE show of my childhood, Full House) will discuss an advancing genomic editing system and the future is holds.
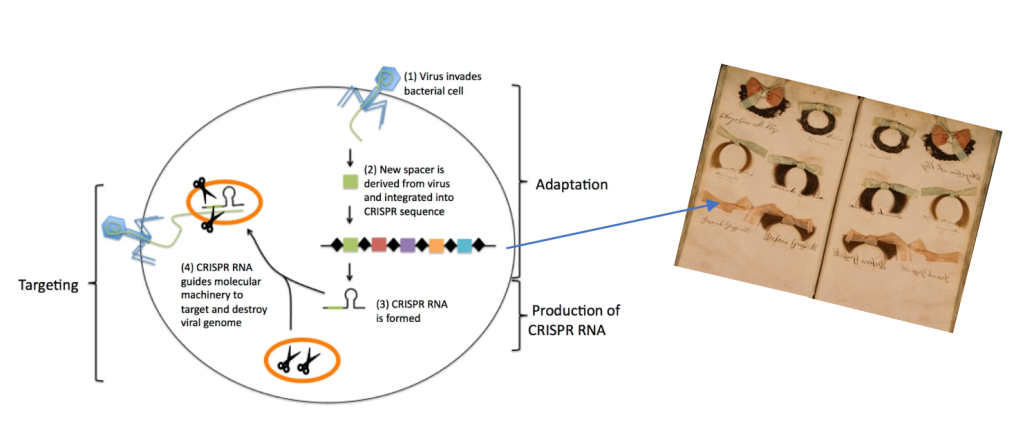
The CRISPR/Cas (clustered regularly interspaced short palindromic repeats-CRISPR associated nucleases) system was originally discovered in many prokaryotes such as most bacteria and Archaea as a form of an acquired immune defense mechanism against foreign DNA, namely viral DNA. Each time a new virus invades a prokaryotic cell, a portion of the virus’s DNA is snipped and incorporate into a specific location within the prokaryote’s genome. In by doing so, the prokaryotic cell is able to detect, respond, and destroy that particular virus if it were to invade the cell again (Zhang et al., 2021). Essentially, think of it as growing scrapbook of snippets of viral “hair”, each belonging to a different virus.
https://sitn.hms.harvard.edu/flash/2014/crispr-a-game-changing-genetic-engineering-technique/
https://www.liveauctioneers.com/item/6958646_364-victorian-hair-work-autograph-album-scrapbook
The finding of this system was merely by accident, occurring in 1987 within the genome of the bacterial species Escherichia coli. Yet, the significance of their finding was yet to be uncovered. Various scientists continued research of the CRIPR/Cas system, piecing together its function and purpose (Zhang et al., 2021). By 2012, two independent laboratories published findings that such system can be carried out in vitro thereby enabling the ability to cut a single piece of targeted DNA with the Cas endoclease enzyme (Gasiuanas et al, 2012; Jinek et al., 2012). This marked the hallmark moment for its use in genome editing.
To date, there have been a variety of Cas enzymes identified in prokaryotes each with slightly different variabilities in their structure and function. The most commonly used and best known is Cas9. The CRISPR/Cas9 system consists of two main parts; the Cas9 enzyme itself that serves as the molecular scissors that which cuts across both strands of DNA and a piece of RNA referred to as the guideRNA (gRNA). The gRNA does just as what the name implies, it guides the Cas9 enzyme complex to the targeted sequence within a genome to be edited. The sequence is carefully created to be complementary to the DNA sequence to be targeted. Delivery of the CRIPSR/Cas9 system can be achieved in one of three ways: DNA plasmids, RNA molecules, or RNA and protein complexes (i.e. gRNA and Cas9). In the event the CRISPR/Cas9 system successfully locates and binds to the targeted genome sequence and makes a double strand cut, one of a two outcomes can result. The first is referred to as a non-homologous end join (EHEJ) where a few random nucleotides are added or deleted at the cut site location. This technique is often used to silence a gene (gene knockout). The alternative is known as a homology-directed repair (HDR) where a DNA template is introduced in addition to the CRISPR/Cas9 system; enabling one to replace an undesirable sequence or introduce an individual gene (gene knock-in). The latter utilizes homologous recombination between the endogenous genome and exogenous DNA template strand (Zhang et al., 2021) See below for a visual of this process.
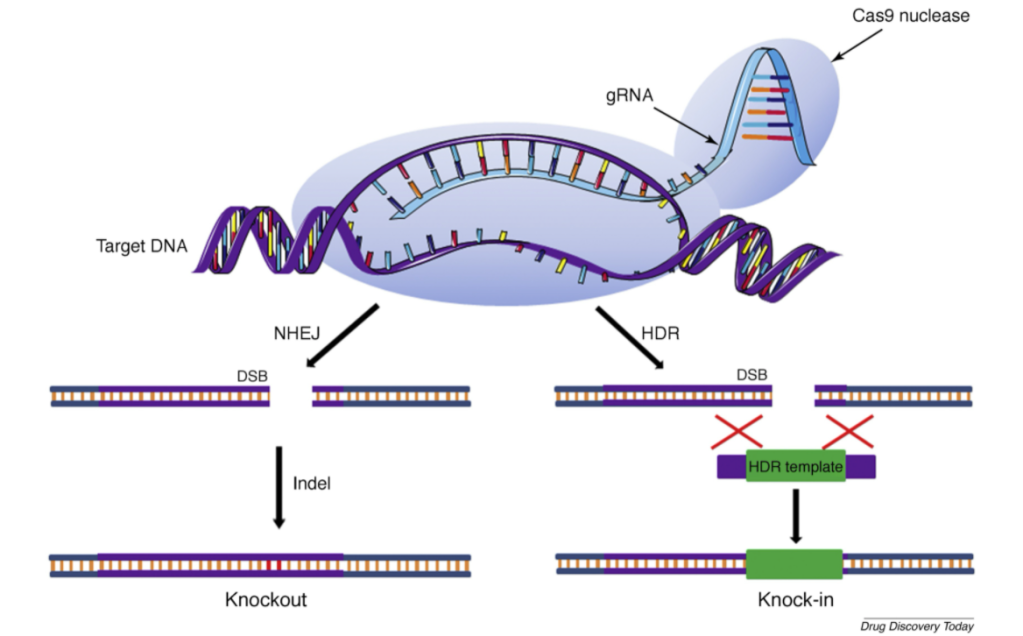
Photo Source: https://www.sciencedirect.com/science/article/pii/S1359644618305245#fig0005
Since 2012, CRISPR/Cas systems have been undergoing much research surrounding its potential use as a treatment route for various diseases including genetic disorders such as sickle cell anemia and cystic fibrosis, various cancers including breast and lung cancer, and HIV.
In March of 2020, Nature published an article about the first person to receive directly an vivo CRISPR/Cas9 treatment in efforts to remove genomic mutations causing a rare condition called Leber’s congenital amaurosis 10 (LCA10). The trial is named BRILLIANCE. Such condition is the leading causes of blindness in children and there is no available cure. The treatment involves the CRISPR/Cas9 system inserted directly into the eye through a viral vector. The gene-editing system is encoded within the virus’s genome. Prior to this method of introduction, clinical trials utilizing CRISPR/Cas gene-editing treatment have been achieved by initial removal of an individual’s cells and genomic editing outside of the body. Treated cells are then reintroduced back into the individual.
Mutation of the gene CEP290 (expressed in photoreceptor cells) is responsible for the onset of this disease and is an ideal candidate for this type of technology. Usually, a healthy gene is swapped out for the disease-causing gene however, CEP290 is quite large thus difficult to fit inside a viral vector. Thus, the mutated gene is deleted instead. The hope is that despite the deletion, the photoreceptor cells are still viable. Thus, is it theorized that vision will improve after receiving treatment.
Although this treatment seems quite promising, off target effects can still occur where the CRISPR/Cas9 system edits a non-targeted sequence within one’s genome. Furthermore, because the CRISPR/Cas9 originates from prokaryotes, the immune system of the host it is introduced into may evoke an immune response against the components (Zhang et al., 2021).
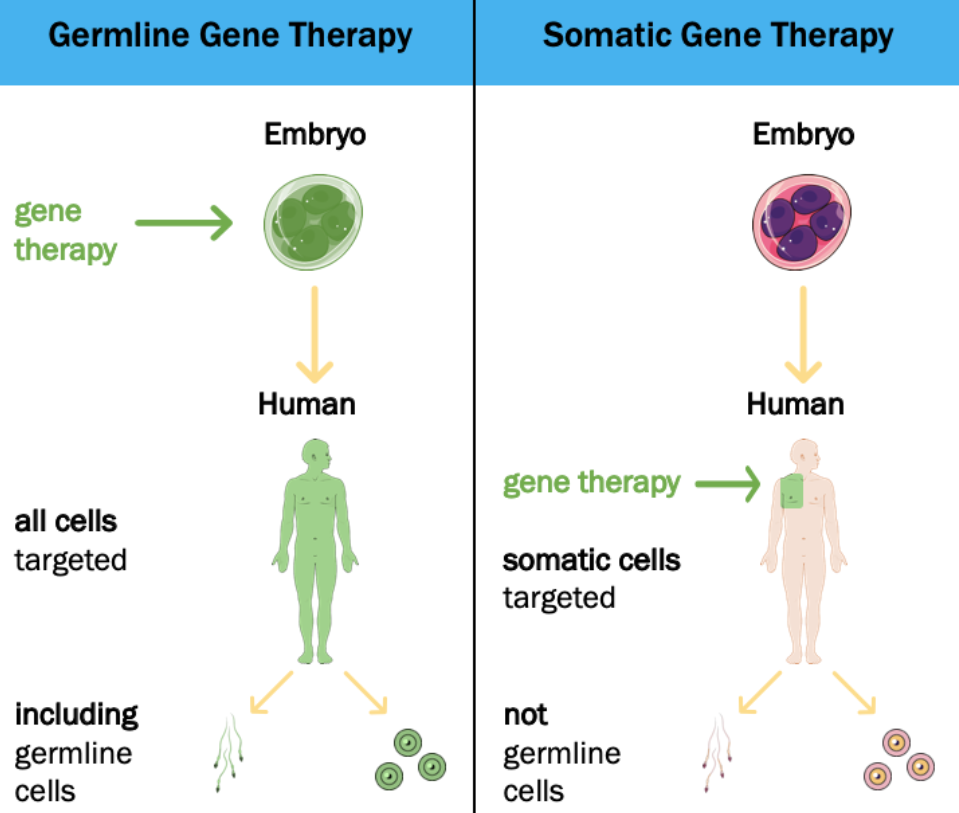
As of now, CRISPR/Cas9 use is limited in that it cannot be applied to human germline or embryonic cells in the United States along with many other countries. Editing these cell’s genome would result in passing edits to future potential offspring of the individual. As mentioned, this system is not perfect where off-target editing can occur. Such poses a major risk in germline and embryonic cells in the event an off-target mutation occurs, as all cells in the organism would carry said mutation. This is in contrast to a mutation introduced by CRISPR/Cas9 in somatic cells where only a localized area of cells would be affected (and not heritable) (Schleidgen et al., 2020; Griffiths et al., 2000). It is also possible that only some cells are “fixed” whilst others remain mutated leaving a “patchwork” or “mosaicism” of normal and mutated cells. When genome-editing is conducted this early in development, there is no way to distinguish between the embryonic cells with the disease carrying genome and the ones without. (Nature, 2019).
In addition, even if CRISPR/Cas systems were faultless, ethical concerns largely question the legality and morality of how germline editing affects future generation, arguing an overstepping of future individual’s autonomy (Habermas, 2003). Modifications that may pose long-term damaging effects in future generations would occur without their consent. Zooming out to the bigger picture, germline cells editing ultimately would affect the human gene pool thereby affecting the whole of humanity (Schleidgen et al., 2020).
In my personal opinion, the future this technology may achieve is both highly impressive and scary. I am still at a tossup over the implications CRISPR/Cas systems hold for therapeutic purposes. On one hand I am supportive in utilizing it for incurable and life-threatening diseases yet on the other hand diseases are quintessentially a form of population control. My fear is that once CRISPR/Cas becomes a regular and perfected technique used to treat diseases, more push will be seen for enhancement and non-therapeutic purposes such as increase intelligence. This would, in my eyes, diminish the beauty of life itself and singularity that lies within each individual ranging from appearance, personality, and capabilities. As technology advancements in medical treatment continues, rather than asking Can we? we are now being forced to ask ourselves Should we?